ACRO released findings from its latest annual survey, the sixth year tracking how the clinical research industry is embracing risk-based quality management (RBQM Solutions). Summary: as older trials wrap up and innovation accelerates, the industry is moving decisively toward more efficient, risk-based approaches including opportunities for RBQM solutions.
Below we summarize the findings of the report. Want the complete picture? Download ACRO’s full RBQM Report Trends in Risk Based Quality Management to explore the latest trends and insights.
Highlights
Based on ACRO’s 2025 landscape survey, risk-based quality management (RBQM) solutions are experiencing robust growth driven by several compelling factors:
Explosive Adoption Rates
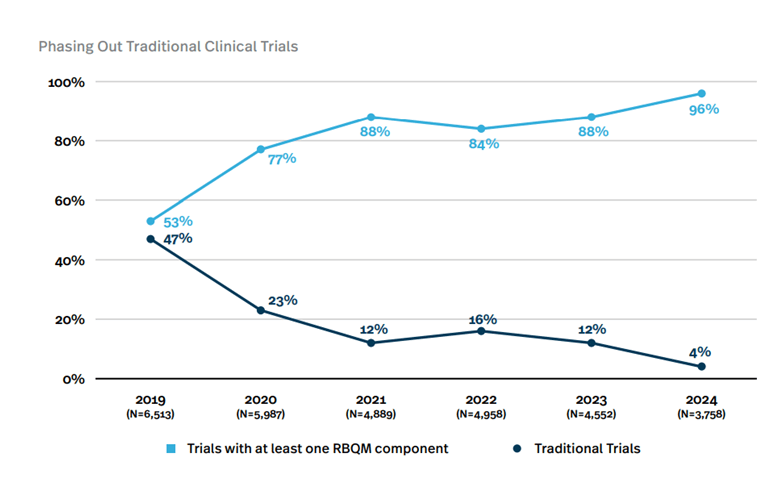
In 2024, 96% of clinical trials included at least one RBQM component. This is a dramatic jump from just 53% in 2019 . Traditional trial models are rapidly phasing out as the industry recognizes the efficiency gains from risk-based approaches.
Regulatory Endorsement
The FDA and EMA have publicly stated that risk-based approaches are the most effective and efficient monitoring methods. They recommending that sponsors use an “end-to-end” risk-based approach in study planning . This regulatory backing is accelerating adoption across the industry.
Data Complexity is Making Traditional Monitoring Obsolete
The volume and complexity of clinical trial data is exploding. Phase III protocols now generate more than 3.5 million data points , and upwards of 70% of data volume is coming from sources outside traditional electronic data capture systems. This includes lab data, wearable devices, ePRO, and eCOA. The manual, on-site monitoring methods used in traditional trials simply can’t keep pace with this data explosion, making centralized monitoring and RBQM essential rather than optional.
The 100% SDR/SDV Model is Broken
Despite RBQM’s proven benefits, many trials still rely on 100% source data review (SDR) and source data verification (SDV). In large studies started in 2024, 100% SDR is being used 82% of the time and 100% SDV is being used 30% of the time . However, experience suggests 100% SDR/SDV leaves more room for errors and opportunities for mistakes while costing the industry significant time, capital, and human resources for little return. This inefficiency creates a massive opportunity for RBQM solutions.
AI and Technology Advancements Are Accelerating the Shift to RBQM Solutions
As the industry moves toward direct data sources and away from manual transcription, the need for SDV will be significantly reduced if not eliminated entirely. Additionally, artificial intelligence is opening new opportunities to maximize accuracy and efficiency in clinical data review, with the FDA leading the way by fully embracing AI.
The Bottom Line
RBQM solutions enable early issue detection, improve data quality, increase patient safety, and reduce unnecessary resource expenditure; while traditional monitoring becomes increasingly impractical. With regulatory support, exploding data complexity, and technological advancement creating a perfect storm, RBQM is transitioning from optional best practice to operational necessity.
Download ACRO’s full RBQM Report Trends in Risk Based Quality Management to explore the latest trends and insights.
About ACRO Health
Founded in 2002, ACRO represents the world’s leading clinical research and technology organizations.
ACRO and its members advocate on a global basis for safe, ethical, high-quality medical research. This helps patients benefit from the development of new treatments and therapies. ACRO member companies employ over 410,000 people worldwide, conduct research in nearly every global region, and generate an estimated $98 billion in revenue per year.
View & research the software vendors providing RBQM software solutions.